

24*7 Hours For Clients
+91-8077848577
+91-9595083550
+91-8077848577
2nd Floor, GMS Arcade, GMS Road, Dehradun, Uttarakhand, India. Pin-248001
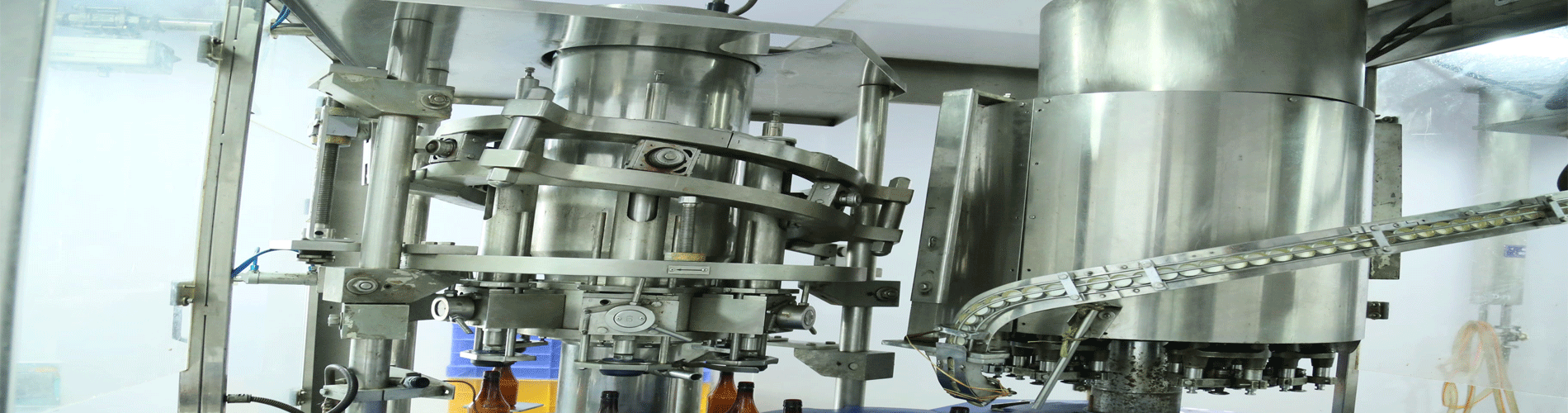
Acies Pharmaceutical Pvt Ltd., is professionally managed with rapidly progressing team engaged in Research and Manufacturing of world class specialty pharmaceutical formulations. Our facilities are WHO-GMP approved, and equipped with the latest state-of-the-art technology to produce products meeting international standards. We welcome our Clients to visit our plants any time.
Acies Pharmaceutical is leading manufacturer and exporter of pharmaceutical, nutraceutical and ayurvedic product. We offer high level of service and provide full balance of value services to our clients both global and domestic. Our team of expert quality analysts conducts the quality survey for each product. We use our richness in domain experience to be perfect in terms of quality, purity, effectiveness and composition. We are able to stand spaced out from our counterpart due to motivation, ethical business policy and industry experience
The unit is situated in a pollution free environment with a few other industrial units in the vicinity and located in Govt. approved Industrial area of district Udham singh nagar (uttarakhand). It can also be reached by air & the nearest airport is Pantnagar at about 30 Kms from the plant. The premises is located, designed, constructed, adapted and maintained to suit the production of Small volume liquid Injectable (Hormones & General Injections), Ophthalmic Products (Eye, Ear Drops), tablets, capsules liquid & External Preparation . Following Good Manufacturing Practices (GMP). The layout and design is aimed to minimize the risk of errors and permit effective cleaning and maintenance in order to avoid contamination, mix-up, build-up of dust or dirt, in general to have a flow pattern of product and raw material.
The quality policy of Acies is outlined in the Quality Manual and in the Quality System. All manufacturing activities of Acies are based on the principles of PIC/S and WHO guidelines and local Drug Regulatory Authority. The Quality Policy of Acies is outlined on the basis of Quality Principles for Acies for Biological & Non-Biological Products countrywide.
